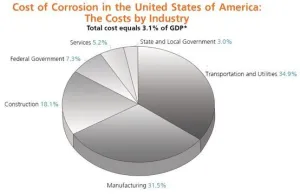
Corrosion is the chemical or electrochemical reaction between a material, most often a metal, and its surrounding environment that results in the gradual deterioration of the material and its properties. In many industrial applications, corrosion occurs when metals react with oxygen and moisture, leading to oxidation.
The most familiar form of corrosion is rust on iron and steel. However, corrosion can take many forms depending on the metal and exposure conditions. These include white rust on galvanized steel, tarnish on copper alloys, pitting on stainless steels, and flaking or scaling on carbon steel surfaces. Over time, it can weaken structural integrity, reduce part performance, and lead to costly failures if not properly controlled.
How corrosion occurs
It typically begins when three elements are present: a metal surface, moisture or an electrolyte, and oxygen or another corrosive agent. Together, these elements create an electrochemical reaction in which metal atoms are gradually converted into more stable compounds such as oxides or salts.
Environmental conditions such as humidity, temperature fluctuations, airborne contaminants, and surface residues can accelerate this process. Poorly controlled conditions can cause metals to corrode, even during indoor storage or transport.
Stop corrosion before it starts
Managing moisture, contaminants, and exposure conditions is critical to corrosion control. ZERUST® specialists can help you break the cycle with proven prevention strategies.
Common causes during indoor storage
Indoor environments are often assumed to be safe for metal parts, yet corrosion can still occur under seemingly controlled conditions.
High relative humidity is one of the most common causes of indoor corrosion. When moisture condenses on metal surfaces, it creates the electrolyte needed to initiate corrosion. Temperature swings between day and night or seasonal changes can worsen this effect by causing repeated condensation cycles.
Airborne contaminants such as chlorides, sulfur compounds, or industrial pollutants can also accelerate corrosion indoors. These contaminants may originate from nearby manufacturing processes, cleaning chemicals, or outside air entering the facility.
Another frequent cause is inadequate packaging or ventilation. Metal parts stored in unprotected cartons, plastic bags without VCI protection, or partially sealed enclosures can trap moisture and contaminants against the surface, increasing risk over time.
Common causes during land and overseas shipping
Corrosion during shipping is often caused by rapidly changing environmental conditions and prolonged exposure to moisture and contaminants while metal parts are in transit.
During land shipping, temperature fluctuations are a primary contributor. As shipments move between regions or experience day and night temperature swings, warm air inside packaging can cool and condense on metal surfaces. This condensation provides the moisture needed to initiate corrosion, especially when parts are packaged without adequate moisture or corrosion control.
Road salts, dust, and airborne industrial pollutants are another major factor in land transportation. These corrosive contaminants can enter packaging through small openings, damaged wraps, or pallet gaps and settle on metal surfaces during transit. In the winter months, salt exposure significantly increases the risk for shipments traveling by truck or rail.
Vibration and handling during transport can also compromise protective packaging. Repeated movement may shift parts, tear packaging materials, or expose bare metal surfaces, allowing corrosion to begin before the shipment reaches its destination.
For overseas and export shipping, corrosion risks are often even greater due to longer transit times and harsher environments. Ocean freight exposes metal parts to high humidity, salt-laden air, and temperature cycling inside shipping containers. Container rain, a common issue during overseas transport, occurs when moist air condenses on the interior walls or ceiling of a container and drips onto cargo below.
Extended storage at ports, customs delays, and intermodal transfers can further increase corrosion exposure by trapping moisture inside packaging for weeks or months. In these conditions, even small amounts of residual moisture or chloride contamination can lead to severe corrosion if not properly managed.
Understanding what causes corrosion during both land and overseas shipping helps manufacturers and exporters implement appropriate packaging and environmental controls to reduce corrosion-related damage, delays, and costly product failures.
Common causes during metalworking and manufacturing
Metalworking operations frequently expose freshly machined or formed surfaces that are highly susceptible to corrosion.
Water-based coolants, cutting fluids, and wash solutions are common contributors. If residues remain on parts after machining or cleaning, they can retain moisture or contain corrosive additives that accelerate oxidation.
Flash rust is another common issue in manufacturing environments. It can occur quickly after machining, rinsing, or pressure washing when bare metal surfaces are exposed to humid air.
Additionally, multimetal contact during assembly or processing can lead to galvanic corrosion. When dissimilar metals are in electrical contact in the presence of moisture, the less noble metal corrodes more rapidly.
Why understanding corrosion is important
Understanding what corrosion is and how it occurs allows manufacturers, shippers, and storage facilities to take proactive steps to prevent damage. Corrosion not only affects the appearance of metal parts but can also compromise dimensional accuracy, mechanical performance, and long-term reliability.
By identifying the environmental and process-related causes of corrosion during storage, shipping, and metalworking, organizations can implement appropriate corrosion prevention strategies, reduce rework and scrap, and extend the usable life of metal components.
Need help mitigating corrosion?
Corrosion can occur during storage, shipping, and manufacturing. ZERUST® experts can help you identify risks and implement proven protection solutions.